The Krebs Cycle is one of the most crucial series of chemical reactions to take place in the human body. The products of the cycle play an essential role in maintaining energy levels and brain health.
If you’re experiencing brain fog, chronic fatigue, or a general lack of energy, the cause could be an inefficiency in your citric acid (Krebs) cycle.
This article provides an in-depth examination of the steps of the cycle, the importance of its products, and whether supplementation of these products could help boost energy levels.
Key Takeaways
- The Krebs Cycle is also known as the citric acid or tricarboxylic acid cycle
- It is the second of a total of three stages in cellular respiration
- The cycle is an aerobic process with nine steps, each controlled by different enzymes
- It turns pyruvate into acetyl CoA, which undergoes several reactions to produce ATP, carbon dioxide, NADH and FADH2
- The TCA cycle is essential for energy production and amino acid and fatty acid synthesis in the body
Krebs Cycle Overview
Also known as the citric acid cycle or tricarboxylic acid cycle, the process is vital to the survival of cells in humans, animals, plants, and fungi. It was named after Hans Adolf Krebs, who discovered and studied the cycle in 1937.
What Is the Krebs Cycle?
The Krebs Cycle is a series of chemical reactions or steps that uses oxygen to produce energy through cellular respiration. It is the second step of three stages involved in cellular respiration.
The Krebs Cycle is sometimes called the citric acid cycle because the chemical formed after the first step is called citric acid.
What Is the Role of the Krebs Cycle?
The Krebs Cycle is responsible for the aerobic respiration of glucose derivatives, fatty acids, and protein to produce energy, along with many other products necessary for building amino acids and adenosine triphosphate [1].
Where Does the Krebs Cycle Take Place?
The Krebs Cycle takes place in the matrix of a cell’s mitochondria. Most of the enzymes required for the cycle to take place are also found in the matrix, while some are located in the inner membrane of the mitochondria.
Krebs Cycle and Brain Health
The citric acid cycle is essential to maintaining brain health and energy levels [2].
Unlike the rest of your body, your brain can and will use lactate as an energy source in the tricarboxylic acid cycle [3]. Besides energy, the most important product of the TCA cycle is hydrogenated NAD or NADH.
Does NAD Heal the Brain?
NADH is one of the final products of the citric acid cycle and is vital to brain health and functioning. NAD+ is a coenzyme that is of critical importance to all body cells, not only the brain.
However, NAD+ can counteract the effects of some neurodegenerative diseases like [4]:
- Alzheimer’s
- Parkinson’s
- Huntington
- Amyotrophic lateral sclerosis
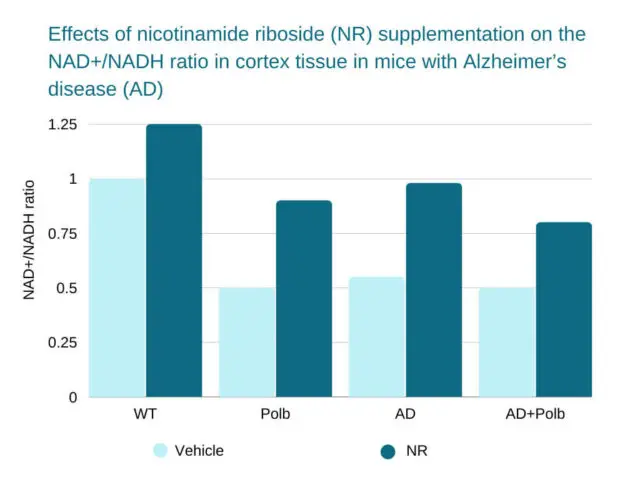
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5828618/
With age, your NAD+ levels naturally decline [5]. Taking an NAD+ supplement can help increase your body’s level of this coenzyme and possibly improve brain health.
Does NAD Help Brain Fog?
Despite brain fog not being a medical diagnosis, it can cause genuine problems for people who suffer from it, including:
- Memory loss
- Feeling spaced out
- Feeling slow
- Confusion
Brain fog is caused by various mental stressors, including anxiety, lack of sleep, depression, and malnutrition.
Due to the positive effect of NAD on brain health and energy levels, taking nicotinamide riboside (NR) or NAD supplements could boost the levels of NAD produced by the citric acid cycle and help combat the symptoms of brain fog, and improve cognition and blood flow to the brain [6].
Let’s take a closer look at the various steps of the Krebs cycle and how NAD, NADH, and NAD+ are formed.
What Are the 9 Steps of the Krebs Cycle?
There is widespread debate over the number of steps involved in the Krebs Cycle. Some incorrectly believe there are only five, while others claim the actual number is eight.
The reasoning behind the eight-step process is because some scientists believe the Krebs Cycle starts when citrate is formed.
Others insist the first step is the oxidative decarboxylation of acetyl CoA. Some scientists believe this is an initial reaction and refer to it as Step 0.
Below are the complete nine Krebs Cycle steps.
1. Oxidative Decarboxylation of Acetyl Coenzyme A
Oxidative decarboxylation is the link between glycolysis and the citric acid cycle. For each glucose molecule undergoing glycolysis, two pyruvate molecules are formed.
Each pyruvate or pyruvic acid molecule, in turn, forms one acetyl coenzyme A, one NADH, and one carbon dioxide molecule, a reaction catalyzed by the pyruvate dehydrogenase complex, which is found in the matrix of the mitochondria [7].
Acetyl CoA moves on to the next step, which is where many believe the Krebs Cycle begins.
|
Reactant(s) |
Enzyme(s) |
Product(s) |
| Pyruvate (pyruvic acid) | Pyruvate dehydrogenase complex | Acetyl CoA, carbon dioxide, NADH |
2. Citrate Formation
Also known as the condensation of acetyl CoA, this step involves the bonding of oxaloacetate, containing four carbon atoms, to the acetyl CoA molecule, which is a two-carbon atom compound.
Oxaloacetate bonds to the acetyl group in acetyl CoA. A water molecule breaks the bond between the acetyl group and the rest of the molecule, and coenzyme A is released from the compound. This step of the reaction is catalyzed by the citrate synthase enzyme [8].
This leaves a molecule of citrate (citric acid), which contains six carbon atoms, as one of the reaction’s products.
|
Reactant(s) |
Enzyme(s) |
Product(s) |
| Acetyl CoA, oxaloacetate, water | Citrate synthase | Citrate (citric acid) |
3. Reshuffling of the Citrate Molecule
A reshuffling reaction involves the dehydration and rehydration of a chemical compound or molecule. The reshuffling of citrate produces an isomer called isocitrate [9].
First, citrate undergoes dehydration to form cis-aconitase. Then, the rehydration of cis-aconitase produces isocitrate. This relocates the -OH group as a water molecule from one part of the citrate molecule to another.
This reaction is reversible and catalyzed by the enzyme aconitase.
|
Reactant(s) |
Enzyme(s) |
Product(s) |
| Citrate | Aconitase | Isocitrate |
4. Oxidative Decarboxylation of Isocitrate
The oxidative decarboxylation of isocitrate is the first of four redox reactions.
Redox reactions involve the gain (reduction) and loss (oxidation) of an electron. In this step, the isocitrate is oxidized, releasing a molecule of carbon dioxide and forming a molecule called alpha-ketoglutarate, containing five carbon atoms [10].
The electron released from the oxidation reaction reduces nicotinamide adenine dinucleotide (NAD+) to form NADH.
The enzyme isocitrate dehydrogenase catalyzes this step.
|
Reactant(s) |
Enzyme(s) |
Product(s) |
| Isocitrate | Isocitrate dehydrogenase | α-ketoglutarate, carbon dioxide, NADH |
5. Oxidative Decarboxylation of α-Ketoglutarate
The second redox reaction in this step oxidizes the alpha-ketoglutarate and reduces another NAD+ ion into NADH + H+. A carbon dioxide molecule is released in the process.
The remaining molecule, containing four carbon atoms, bonds with coenzyme A to form succinyl CoA.
The reaction is catalyzed by a complex of 24 enzymes known as α-ketoglutarate dehydrogenase [11].
|
Reactant(s) |
Enzyme(s) |
Product(s) |
| α-ketoglutarate | α-ketoglutarate dehydrogenase | Succinyl CoA, carbon dioxide, NADH, H+ |
6. Oxidative Phosphorylation of Adenosine Diphosphate (ADP) or Guanosine Diphosphate (GDP)
The phosphorylation of ADP or GDP occurs when the link between the CoA group in succinyl CoA is broken and releases enough energy to add an inorganic phosphate group to the ADP or GDP, producing either adenosine triphosphate (ATP) or guanosine triphosphate (GTP), respectively.
The split between succinyl CoA and its CoA group forms succinate.
This reaction is catalyzed by the enzyme succinyl-CoA synthase [12].
|
Reactant(s) |
Enzyme(s) |
Product(s) |
| Succinyl CoA | Succinyl-CoA synthase | Succinate, ATP/GTP |
7. Dehydrogenation of Succinate
The dehydrogenation of succinate forms a molecule called fumarate. This is the only dehydrogenation/oxidation process in the Krebs Cycle where NAD+ isn’t involved.
Instead, flavin adenine dinucleotide (FAD) acts as an electron carrier and hydrogen acceptor to form FADH2. The enzyme responsible for catalyzing this reaction is succinate dehydrogenase and is found in the mitochondrial inner membrane.
In some cases, the two hydrogen atoms attach to a carrier called ubiquinone to form ubiquinol instead of the flavin adenine dinucleotide. Either ubiquinol or FADH2 carries electrons to the electron chain and eventually enables the production of two ATP molecules [13].
|
Reactant(s) |
Enzyme(s) |
Product(s) |
| Succinate | Succinate dehydrogenase | Fumarate |
8. Hydration of Fumarate
In a reversible reaction, fumarate is hydrated to form a four-carbon molecule called L-malate. This step is catalyzed by fumarate hydratase (fumarase) [14].
|
Reactant(s) |
Enzyme(s) |
Product(s) |
| Fumarate | Fumarate dehydrase (fumarase) | L-malate |
9. Oxidation/Dehydrogenation of L-Malate
The last step of the citric acid cycle involves the oxidation of L-malate to regenerate oxaloacetate. The reaction is reversible and catalyzed by L-malate dehydrogenase, which is found in the matrix of the mitochondria [15].
L-malate is oxidized, and NAD+ is reduced to form NADH.
The formation of oxaloacetate both completes and enables the cycle to start again. The NADH produced through reduction can participate in the oxidative phosphorylation of step six.
|
Reactant(s) |
Enzyme(s) |
Product(s) |
| L-malate | L-malate dehydrogenase | Oxaloacetate |
Citric Acid Reactions Overview
Each glucose molecule produces two pyruvic acid molecules. That means one glucose molecule is responsible for two turns of the cycle.
Acetyl CoA + 3NAD+ + ubiquinone + GDP or ADP + phosphate + two water molecules → CoA-SH + 3NADH + 3H+ + QH2 + GTP or ATP + two carbon dioxide molecules
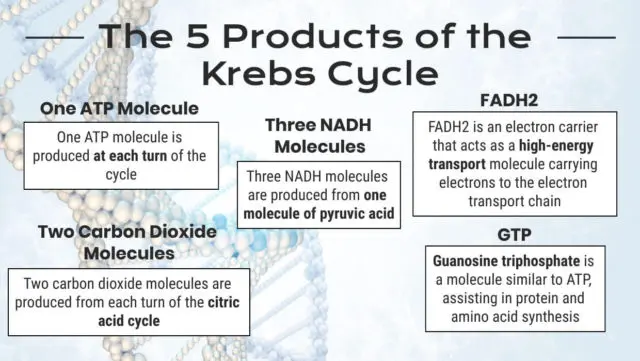
What Are the Products of the Krebs Cycle?
Each of the citric acid cycle products plays an essential role in bodily functions or other chemical reactions.
The most significant Krebs Cycle products formed by each turn of the cycle are listed below:
GTP
Guanosine triphosphate is a molecule similar to ATP that assists in protein and amino acid synthesis [16].
Although ATP and GTP have two closely linked pathways, the specific metabolic pathway of GTP is unique to the molecule.
One ATP Molecule
One ATP molecule is produced at each turn of the cycle. That means two ATP molecules are produced for each glucose molecule that enters glycolysis.
Adenosine triphosphate is the primary energy carrier in your body. When phosphate is removed from the molecule, energy is released, and the molecule is now called adenosine diphosphate (ADP)
Your body needs to produce ATP to provide energy for several functions, including [17]:
- Muscle contractions
- Ion movement
- Chemical synthesis
- DNA/RNA synthesis
Each aerobically respired glucose molecule produces 36 ATP molecules [18].
Three NADH Molecules
Three NADH molecules are produced from one molecule of pyruvic acid. NADH is critical for energy metabolism and gene expression [19].
NADH is often prescribed to treat symptoms of chronic fatigue syndrome, including [20]:
- Prolonged fatigue
- Weakness
- Low-grade fever
- Headaches
- Difficulty sleeping
FADH2
FADH2 is an electron carrier that acts as a high-energy transport molecule carrying electrons to the electron transport chain [21].
Two Carbon Dioxide Molecules
Two carbon dioxide molecules are produced from each turn of the citric acid cycle.
Carbon dioxide levels in the body must be regulated as the molecules play an essential role in blood pH maintenance, respiratory drive, and the affinity of the hemoglobin in your red blood cells for oxygen [22].
Excess carbon dioxide is expelled through your lungs when you exhale.
FAQ
Below are some of the most commonly asked questions about the TCA cycle.
What Is the Difference Between the TCA Cycle, Citric Acid Cycle, and Krebs Cycle?
There is no difference between the three. The TCA cycle, citric acid cycle, and Krebs Cycle are all different names for the same process that uses pyruvic acid molecules to produce ATP or GTP that provides our body with energy.
What Is the Krebs Cycle in Simple Terms?
The citric acid cycle is an integral part of, and the second step in, cellular respiration.
It uses pyruvic acid molecules, converts them to acetyl CoA molecules, and then inputs them into a series of reactions that eventually produce ATP or GTP, carbon dioxide molecules, NADH, and FADH2.
What Is the Main Function of the Krebs Cycle?
The primary function of the citric acid cycle is cellular respiration. The ATP produced by the cycle contains three phosphates. When one of the phosphates is removed from the molecule to form ADP, energy is released.
This energy is what allows the cells in our bodies to survive and keep doing their jobs.
Other intermediate products the Krebs Cycle produces also play essential roles in:
What Are the Three Steps of Cellular Respiration?
The three steps of cellular respiration are:
- Glycolysis: Involving reactions that turn one molecule of glucose into a pair of pyruvate molecules. This process also results in producing two ATP molecules and reducing NAD+ to NADH.
- TCA Cycle: The pyruvate molecules go through a series of enzyme-controlled chemical reactions to produce one ATP molecule, three NADH, one FADH2, and two carbon dioxide molecules for each pyruvate molecule that enters the cycle.
- Electron Transport: Here, the reduced NADH and FADH2 are oxidized and donate their electrons to the electron transport chain. As electrons move along the chain, the energy produced is used to fuel another process that eventually results in the production of up to 32 ATP molecules. The oxidized NAD+ and FAD are made available to participate in the next turn of the cycle.
What Does Citric Acid Do to Your Body?
Citric acid is not only produced during the TCA cycle; it can also be found in many foods like citrus fruits.
Besides its role in the TCA cycle, citric acid also provides several benefits to your body, including:
- Promoting the absorption of nutrients like calcium, magnesium, and phosphorus [25]
- The prevention and treatment of kidney stones [26, 27]
However, too much citric acid in your body could cause adverse side effects, including [27]:
- Joint pain and swelling
- Muscle pain
- Abdominal discomfort
- Stiffness
- Mental fatigue
How Do You Make Citric Acid?
Citric acid can be found in a variety of foods, especially fruits, including [28]:
- Lemons
- Grapefruits
- Tangerines
- Limes
- Oranges
Eating a lot of these fruits will naturally increase the concentration of citric acid in your body.
Another crucial source of citric acid is the citric acid cycle, where it’s one of the first chemicals created through the reactions in the cycle.
Conclusion
Despite the Krebs Cycle being a vital part of the body’s energy production process, very few people have a working knowledge of its reactions.
Although it appears complex at first, understanding each step of the process could help you get a better idea of how your body functions, produce energy and help you identify the cause of several adverse symptoms like chronic fatigue and forgetfulness.
References:
- M;, Akram. “Citric Acid Cycle and Role of Its Intermediates in Metabolism.” Cell Biochemistry and Biophysics, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/24068518/.
- Mishkovsky, Mor, et al. “In Vivo Detection of Brain Krebs Cycle Intermediate by Hyperpolarized Magnetic Resonance.” Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism, Nature Publishing Group, Dec. 2012, www.ncbi.nlm.nih.gov/pmc/articles/PMC3519415/.
- Gallagher, Clare N., et al. “Human Brain Utilizes Lactate via the Tricarboxylic Acid Cycle: a 13C-Labelled Microdialysis and High-Resolution Nuclear Magnetic Resonance Study.” OUP Academic, Oxford University Press, 20 Aug. 2009, academic.oup.com/brain/article/132/10/2839/331774.
- Lautrup, Sofie, et al. “NAD+ in Brain Aging and Neurodegenerative Disorders.” Cell Metabolism, U.S. National Library of Medicine, 1 Oct. 2019, www.ncbi.nlm.nih.gov/pmc/articles/PMC6787556/.
- JA;, McReynolds MR;Chellappa K;Baur. “Age-Related NAD + Decline.” Experimental Gerontology, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/32097708/.
- “The Effects of Nicotinamide Adenine Dinucleotide (NAD) on Brain Function and Cognition – Full Text View.” The Effects of Nicotinamide Adenine Dinucleotide (NAD) on Brain Function and Cognition – Full Text View – ClinicalTrials.gov, clinicaltrials.gov/ct2/show/NCT02942888.
- T;, Ogata M;Yagi. “Pyruvate Dehydrogenase and the Path of Lactate Degradation in Desulfovibrio Vulgaris Miyazaki F.” Journal of Biochemistry, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/3023304/.
- La Cognata U;Landschütze V;Willmitzer L;Müller-Röber B; “Structure and Expression of Mitochondrial Citrate Synthases from Higher Plants.” Plant & Cell Physiology, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/8979399/.
- O;, Krebs Ha;holzach. “The Conversion of Citrate into Cis-Aconitate and Isocitrate in the Presence of Aconitase.” The Biochemical Journal, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/13018271/.
- RF;, Colman. “Mechanisms for the Oxidative Decarboxylation of Isocitrate: Implications for Control.” Advances in Enzyme Regulation, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/1977/.
- JP;, Sheu KF;Blass. “The Alpha-Ketoglutarate Dehydrogenase Complex.” Annals of the New York Academy of Sciences, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/10672230/.
- Phillips D;Aponte AM;French SA;Chess DJ;Balaban RS; “Succinyl-CoA Synthetase Is a Phosphate Target for the Activation of Mitochondrial Metabolism.” Biochemistry, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/19527071/.
- S;, Wang Y;Hekimi. “Understanding Ubiquinone.” Trends in Cell Biology, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/26827090/.
- Ajalla Aleixo MA;Rangel VL;Rustiguel JK;de Pádua RAP;Nonato MC; “Structural, Biochemical and Biophysical Characterization of Recombinant Human Fumarate Hydratase.” The FEBS Journal, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/30761759/.
- M;, Minárik P;Tomásková N;Kollárová M;Antalík. “Malate Dehydrogenases–Structure and Function.” General Physiology and Biophysics, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/12537350/.
- W;, Weller M;Haag M;Laing. “GTP, as Well as ATP, Can Act as a Substrate for the Intrinsic Protein Kinase Activity of Synaptic Plasma Membranes.” Molecular and Cellular Biochemistry, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/7311972/.
- MH;, Dunn J;Grider. “Physiology, Adenosine Triphosphate.” National Center for Biotechnology Information, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/31985968/#:~:text=ATP%20is%20consumed%20for%20energy,a%20high%20demand%20for%20ATP.
- Melkonian, Erica A. “Biochemistry, Anaerobic Glycolysis.” StatPearls [Internet]., U.S. National Library of Medicine, 1 Oct. 2020, www.ncbi.nlm.nih.gov/books/NBK546695/.
- W;, Ying. “NAD+ and NADH in Cellular Functions and Cell Death.” Frontiers in Bioscience : a Journal and Virtual Library, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/16720381/.
- Forsyth LM;Preuss HG;MacDowell AL;Chiazze L;Birkmayer GD;Bellanti JA; “Therapeutic Effects of Oral NADH on the Symptoms of Patients with Chronic Fatigue Syndrome.” Annals of Allergy, Asthma & Immunology : Official Publication of the American College of Allergy, Asthma, & Immunology, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/10071523/.
- Cooper, Geoffrey M. “The Mechanism of Oxidative Phosphorylation.” The Cell: A Molecular Approach. 2nd Edition., U.S. National Library of Medicine, 1 Jan. 1970, www.ncbi.nlm.nih.gov/books/NBK9885/.
- Patel, Shivani. “Physiology, Carbon Dioxide Retention.” StatPearls [Internet]., U.S. National Library of Medicine, 4 Jan. 2021, www.ncbi.nlm.nih.gov/books/NBK482456/#:~:text=CO2%20plays%20various%20roles%20in,normal%20levels%20are%20not%20maintained.
- Berg, Jeremy M. “Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways.” Biochemistry. 5th Edition., U.S. National Library of Medicine, 1 Jan. 1970, www.ncbi.nlm.nih.gov/books/NBK22459/.
- Kelly, David J. “The Citric Acid Cycle and Fatty Acid Biosynthesis.” Helicobacter Pylori: Physiology and Genetics., U.S. National Library of Medicine, 1 Jan. 1970, www.ncbi.nlm.nih.gov/books/NBK2413/.
- NII, Yoshitaka, et al. “Effect of Citrus Fruit (Sudachi) Juice on Absorption of Calcium from Whole Small Fish in Healthy Young Men.” Food Science and Technology Research, Japanese Society for Food Science and Technology, 15 May 2007, www.jstage.jst.go.jp/article/fstr/12/1/12_1_27/_article/-char/en.
- LA;, Kern A;Grimsby G;Mayo H;Baker. “Medical and Dietary Interventions for Preventing Recurrent Urinary Stones in Children.” The Cochrane Database of Systematic Reviews, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/29117629/.
- Phillips R;Hanchanale VS;Myatt A;Somani B;Nabi G;Biyani CS; “Citrate Salts for Preventing and Treating Calcium Containing Kidney Stones in Adults.” The Cochrane Database of Systematic Reviews, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/26439475/.
- Abdel-Salam, Omar M E, et al. “Citric Acid Effects on Brain and Liver Oxidative Stress in Lipopolysaccharide-Treated Mice.” Journal of Medicinal Food, Mary Ann Liebert, Inc., May 2014, www.ncbi.nlm.nih.gov/pmc/articles/PMC4026104/.